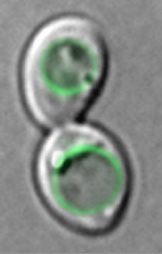
 In this novel study entitled “Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystonosis therapy“, conducted in collaboration with B. Gasnier (Descartes University, Paris), we report the characterization three novel yeast transporters of the vacuolar membrane (Ypq1, -2, -3) involved in cationic amino acid homeostasis. These yeast Ypq proteins turned out to be very similar in sequence to PQLC2, a mammalian protein of unknown function. Detailed study of this protein in B Gasnier’s lab revealed that it corresponds to the long sought lysosomal exporter of cationic amino acids (lysine, arginine, histidine). When expressed in yeast, PQLC2 is localized at the vacuolar membrane (see figure, obtained by E Llinares, showing the PQLC2-GFP protein in two yeast cells) and complements the Ypq-deficiency-associated phenotypes.
In this novel study entitled “Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystonosis therapy“, conducted in collaboration with B. Gasnier (Descartes University, Paris), we report the characterization three novel yeast transporters of the vacuolar membrane (Ypq1, -2, -3) involved in cationic amino acid homeostasis. These yeast Ypq proteins turned out to be very similar in sequence to PQLC2, a mammalian protein of unknown function. Detailed study of this protein in B Gasnier’s lab revealed that it corresponds to the long sought lysosomal exporter of cationic amino acids (lysine, arginine, histidine). When expressed in yeast, PQLC2 is localized at the vacuolar membrane (see figure, obtained by E Llinares, showing the PQLC2-GFP protein in two yeast cells) and complements the Ypq-deficiency-associated phenotypes.
A link to cystinosis. The yeast Ypq proteins and the mammalian PQLC2 belong to the PQ-loop family of transmembrane transporters. Only one PQ-loop transporter, named cystinosin, had been characterized before. This human protein exports cystine (a mixed cysteine) from the lysosome. Mutations in the cystinosin gene cause cystinosis, a rare genetic disease. Cystinotic patients accumulate excess cystine in the lysosomes thus causing renal dysfunctions. These patients are treated with cysteamine, a compound reacting with cystine and converting it into a lysine-like compound efficiently exported from the lysosome. The just published article also shows that PQLC2 is the protein exporting this lysine-like molecule. This opens novel perspectives of research on cystinosis and its therapy by cysteamine.
Leave a Reply