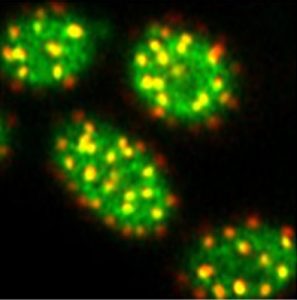
The plasma membrane of cells is known to be compartmentalized into domains enriched in specific lipids and proteins. The best studied membrane domains found in yeast is the “Eisosome Membrane Compartment” (EMC) accumulating diverse proteins including several nutrient transporters. However, how and why these transporters preferentially partition in these EMCs remains unknown.
In a novel study published in PNAS and mainly conducted by Christos Gournas (FNRS postdoc), we report that segregation of the Can1 arginine transporter into EMCs is dictated by its conformation and requires proper biogenesis of sphingolipids. Furthermore, this clustering confers to Can1 and other transporters protection from ubiquitin-dependent endocytosis. This protective role is particularly pronounced under nutrient starvation conditions, where EMCs increase in size and number. It allows cells to preserve a fraction of their nutrient transporters from bulk endocytosis occuring in parallel with autophagy, and to more efficiently resume growth when replenishing compounds are available. This work reveals nutrient-regulated protection from endocytosis as an important role for protein partitioning into membrane domains. It also suggests that EMC-like microdomains existing in human cells could ensure a similar function. This study was supported by the FNRS and conducted in collaboration with the Centre for Microscopy and Molecular Imaging (CMMI) of the Biopark and with Dr. D. Tyteca (UCL, De Duve Institute).
Leave a Reply